Is Manganese a Cation or Anion
It is also strange that a manganese forms a ppt with the Permanganate anion same element in cation anion 14. National Drug Code NDC Directory 7 Pharmacology and Biochemistry 71 FDA Pharmacological Classification FDA Pharm Classes 72 Human Metabolite Information 721 Tissue Locations Basal Ganglia Brain Hair Liver Human Metabolome Database HMDB.

Common Cations Anions Acids Salts And Hydrate Nomenclature Chart Download Printable Pdf Templateroller
For example sodium always forms a 1 cation and magnesium always forms a 2 cation.
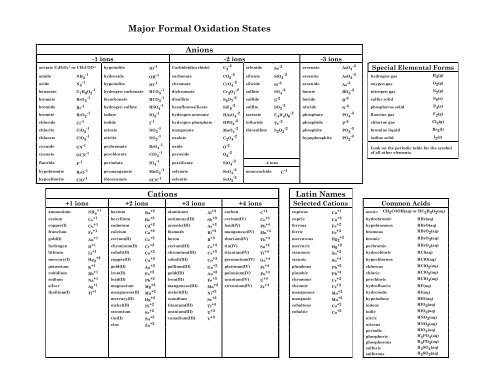
. Give either the name or formula with the correct charge for each of the cations. Anion exchange devices remove anions negatively charged ions like arsenic and nitrate and replace them with chloride. In between the electrodes is an electrolyte liquid or gel that contains charged particles ions.
It is a divalent metal cation a manganese cation and a monoatomic dication71FDA Pharmacological Classification. Ammonium NH4 calcium Ca2 copper Cu2 magnesium Mg2 manganese Mn2 potassium K and zinc Zn2. This is a great test for Manganese ions as the permanganate rarely forms ppts.
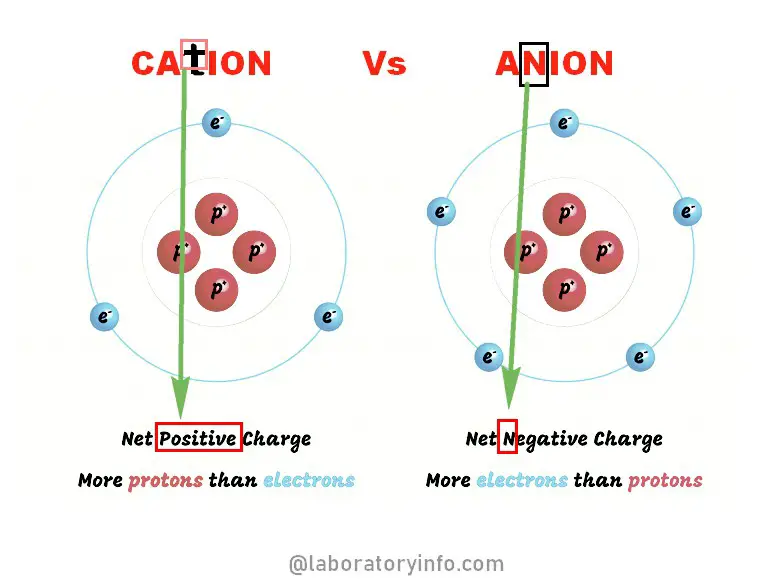
An easy way of remembering whether an element is a cation or anion is that cations are metallic elements and anions are non-metallic. Chlorine Cl- Anion Manganese Mn2 Cation. It has a role as a cofactor.
Sodium Tungstate a A THICK WHITE ppt was formed b No further changes on heating. Boron is an element and is neither an anion or cation. Anion anode cathode cation electron lattice energy negatively - charged ion nuclear charge polarity positively charged ion proton valence orbitals.
ManganeseIIIII oxide is the chemical compound with formula Mn 3 O 4. Ions are atoms or molecules which have gained or lost one or more valencee electron giving the ion a net positive or negative charge. Cations and anions are both ions.
As this ionic substance reacts with the electrodes it generates electrical current. Electrolytic manganese dioxide has little semiconductor-like properties implying that the electron in the matrix is delocalized to some extent and if there is a trivalent manganese cation it has a possibility to. In this regard how do you find the charge of cations.
Some metals always form the same type of cation. They treated the solid manganese dioxide medium as a homogeneous medium composed of cations of MnIV and MnIII and oxide and hydroxyl anions Figure 2. Which are then lost by leaching.
Manganese is a chemical element with the symbol Mn and atomic number 25. If ionized it would tend to from B3 and would be thus a cation. A typical mixture would be 60 percent cation exchange material and 40 percent anion exchange material.
Soils with net positively charged colloids adsorb anions like phosphate sulphate etc. And repel cations like calcium magnesium etc. Also the left side of the periodic table will mostly be cations and the right side is primarily anions.
Draganjacs Introduction to Chemistry CHEM1003 General Chemistry I CHEM1013 and General Chemistry II CHEM1023 classes are responsible for learning the names and formulae for the common acids and common reagents and for learning the names formulae and the charges for. Cation Anion List Worksheet for naming ions Students enrolled in Dr. Current Location Formulas in Chemistry Chemistry Basics Cations and Anions Cations and Anions Dont forget to try our free app - Agile Log which helps you track your time spent on various projects and tasks.
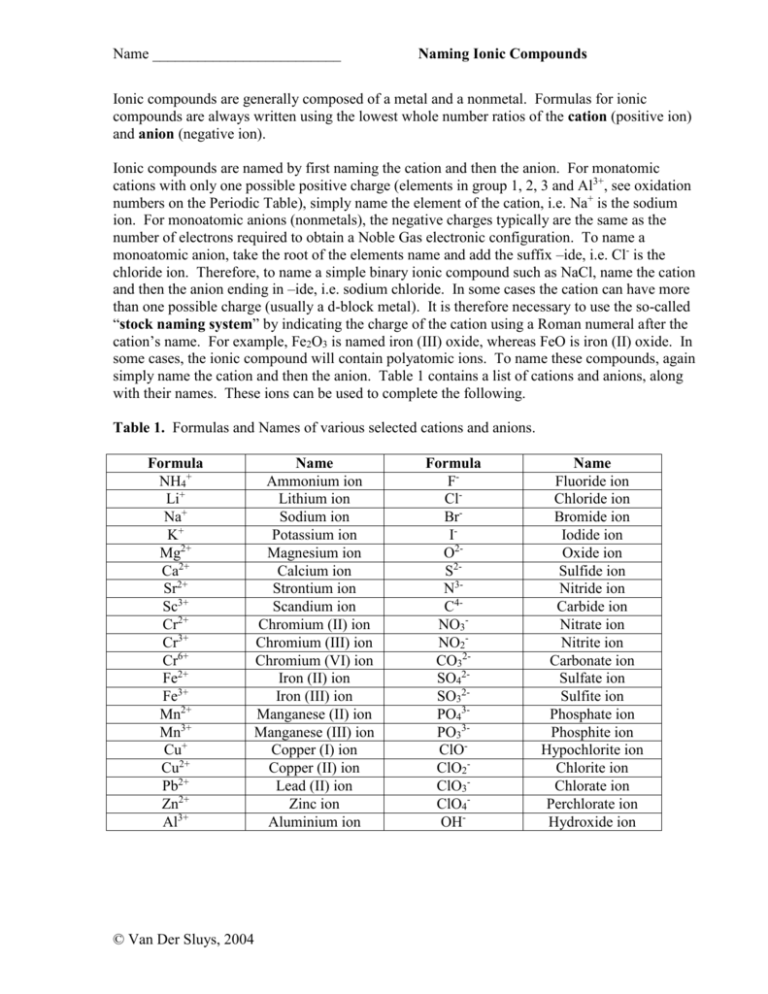
Is Potassium a cation or anion. Common Cations and Anions Name Formula Charge Name Formula Charge Name Formula Charge aluminum Al 3 3 magnesium Mg 2 2 carbonate CO 3 2 2 ammonium NH 4 1 manganese II Mn 2 2 chlorate ClO 3 1 barium Ba 2 2 manganese III Mn 3 3 chloride Cl 1 cadmium Cd 2 2 mercury I. Anions on the other hand gain new ones to become larger in size and have a positive charge.
If a metal element forms an ion it always forms a cation. Mn 3 O 4 is found in nature as the mineral hausmannite. Anion exchange capacity of the soil is usually very low ie a few tenth of a mill equivalent per 100gms of a soil.
Mixed media ion exchange units remove both cations and anions. The difference between a cation and an anion is the net electrical charge of the ion. ManganeseIII Mn3 4 tinIV Sn4 nickelIV Ni4 leadIV Pb4 Roman numeral notation indicates charge of ion when element commonly forms more than one ion.
Manganese is present in two oxidation states 2 and 3 and the formula is sometimes written as MnOMn 2 O 3. In single use dry cell batteries zinc is commonly used as the anode whilst manganese dioxide is a popular choice for the electrolyte cathode. A cationis a positively charged ion and most inorganic chemical contaminants are cations such as.
MANGANESE CATION 2 is an active ingredient in the products Rhodochrosite and Rhodonite. Magnanese III or manganous cation Mn 3 Chromium III or chromous cation Cr 3 Cations with oxidation number greater than 3 Lead IV or lead cation Pb 4 Manganese IV or manganic cation Mn 4 Chromium VI or chromic Cr cation 6 Manganese VII or permanganic Mn cation 7 Tin IV or stannic Sn cation 4. Cations are smaller than the parent atom because theyve lost electrons.
It is a hard brittle silvery metal often found in minerals in combination with iron. To find the ionic charge of an element youll need to consult your Periodic Table. IronIII a 3 charge.
Manganese is a transition metal with a multifaceted array of industrial alloy uses particularly in stainless steels. Is mn a cation or anion. It improves strength workability and resistance to wear.
For example ironII has a 2 charge. Manganese 2 is a divalent metal cation in which the metal is manganese. Anions 1-acetate C 2 H 3 O 2.
Anion exchange capacity increases with the decrease in soil pH.
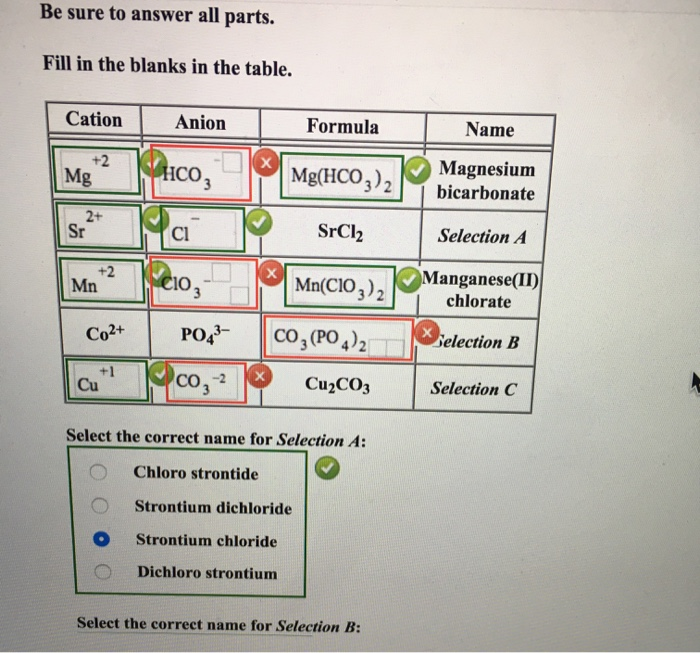
Solved Be Sure To Answer All Parts Fill In The Blanks In Chegg Com

Collection Of Writing Chemical Formulas For Compounds Worksheet Writing Worksheets Chemistry Worksheets Covalent Bonding Worksheet
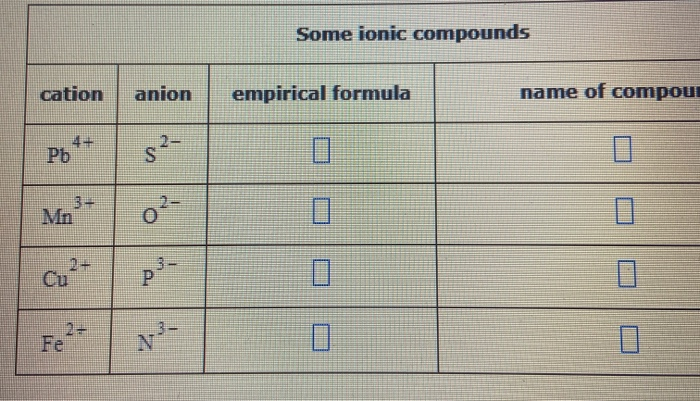
Solved Some Ionic Compounds Cation Anion Empirical Formula Chegg Com

What Is The Difference Between A Cation And An Anion Brushing Teeth Electricity Different
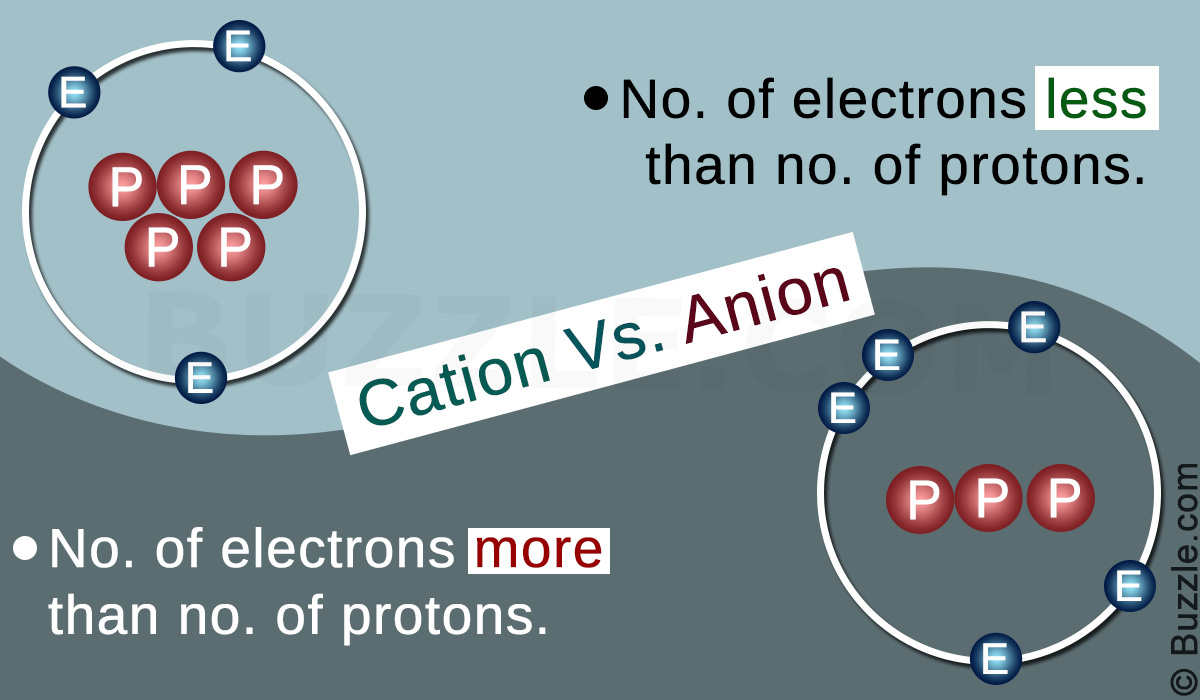
The Basic Difference Between A Cation And An Anion Science Struck
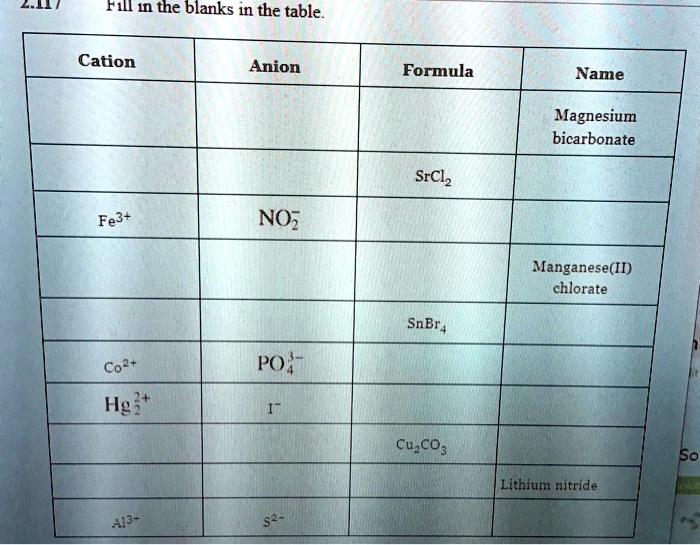
Solved Fil In The Blanks In The Table Cation Anion Formula Name Magnesium Bicarbonate Srclz Fe3 No Manganese Ii Chlorate Po Hg Cuzco3 50 Lithium Nitride 437 Snbr Co
Naming Monatomic Ions And Ionic Compounds Article Khan Academy

Physical Properties Major Cations Anions Silica And Uranium In Download Table

Aleks Deducing The Ions In A Polyatomic Ionic Compound From Its Empirical Formula Youtube

Solved Complete The Table Below By Writing The Symbols For The Cation And Anion That Make Up Each Ior Has Been Completed For You Ionic Compound Cation Anion Nacl Fef Nico Ni

Manganese Cation An Overview Sciencedirect Topics
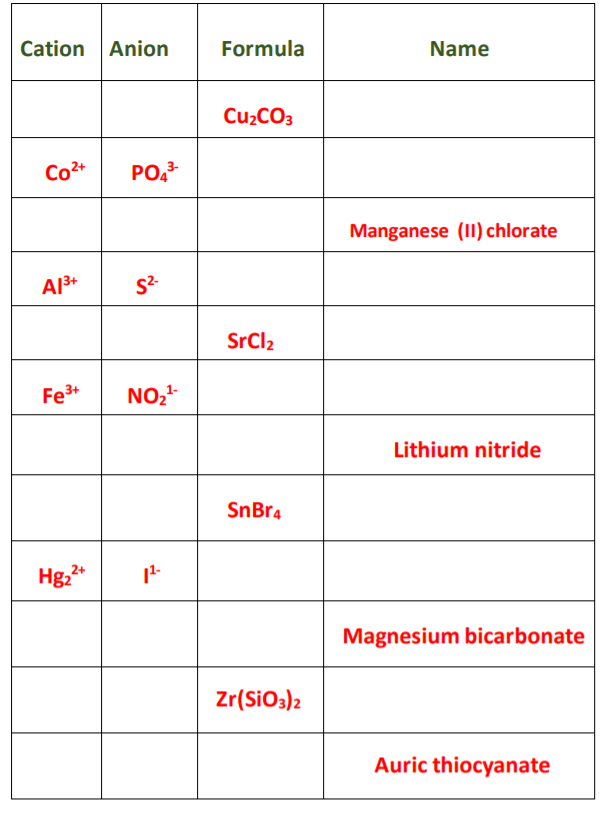
Solved Cation Anion Formula Name Cu2co3 Co2 Po3 Manganese Chegg Com
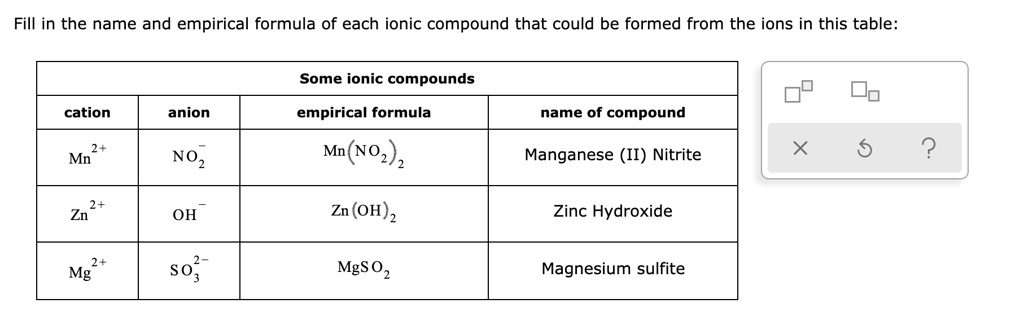
Solved Fill In The Name And Empirical Formula Of Each Ionic Compound That Could Be Formed From The Ions In This Table Some Ionic Compounds Cation Anion Empirical Formula Name Of Compound Mn

Cation Anion Sheet Milwaukie High School
Difference Between Cation And Anion Laboratoryinfo Com

Solved 2 Complete The Table Below With The Proper Ions Chemical Formulas And Compound Names The First Row Is Completed As An Example Cation Anion Chemical Formula Compound Name Nat Ci Nacl Sodium

Periodic Table And Valence Electron Quiz In 2022 Periodic Table Physical Science Electrons
Comments
Post a Comment